Last night, I was reading a fascinating article: some Swedish scientists found a new way to treat blindness. Well, one type of blindness. They did it by using a bioengineered organic material. Because the article is somewhat dense, I thought we could go through it together. The article goes like this:
Act #1: the problem
As you may know, not all blind people have the same condition. There are several disorders that cause blindness, depending on which part of your eye is damaged.
Let’s start then by looking at how the eye works.
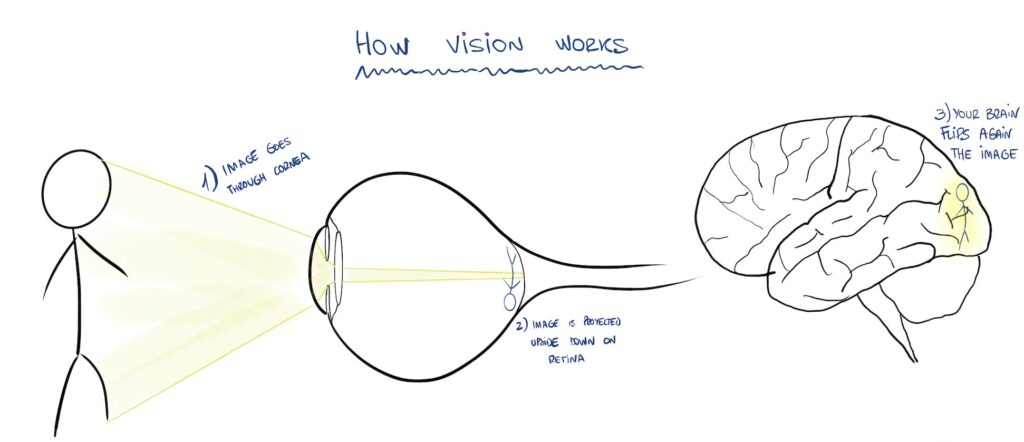
Every object reflects light. Depending on its size, shape and color, the reflected light will be different, which allows us to proyect an image of a distant object (like in a cinema screen).
The reflected light travels and enters our eye, going through our cornea, lens and then it projects into our retina, where an image of the object forms.
That image travels through our optic nerve into our brain, who makes some “image corrections” and voilà, we are now seeing the object.
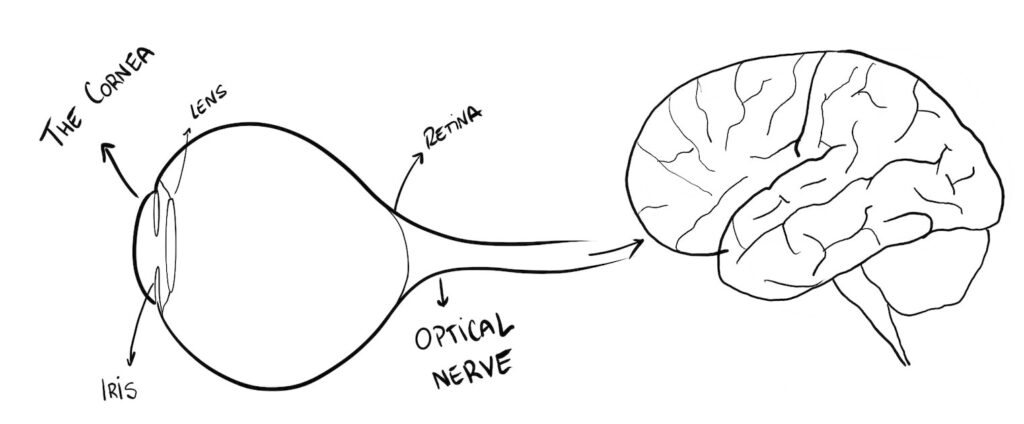
In this study, we have to focus on the cornea.
The cornea is the transparent front part of the eye, the “window” through which light enters our eye. It is like a transparent curved glass.
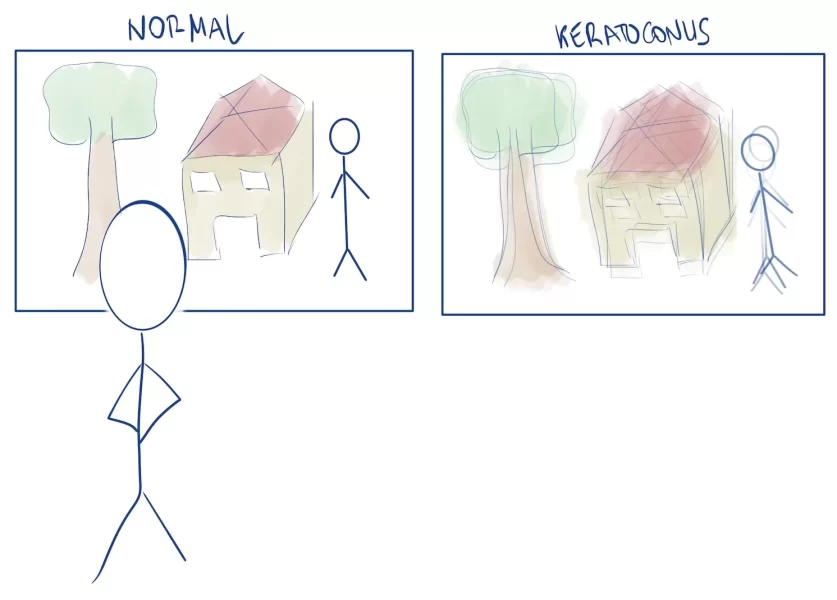
To distribute light evenly, the cornea needs to be homogeneous in its thickness and curvature. If the cornea loses its normal shape, the image we see will get distorted. And that is what happens to these patients.
Our patients suffer from keratoconus, which means their cornea is thinner than normal, and has a cone shape, instead of rounded. Corneas like these are not good for their mission, directing light.
Imagine looking through a window and the glass is distorted: you will see blurry outside. Not only that but also, because their eye surface is deformed, most patients with a severe disease won’t tolerate contact lenses.
Even if you haven’t heard of it, keratoconus is a common disease. Every year, over 1 million people in the world go blind because of corneal disorders (keratoconus and others).
The greatest number of cases happen in low and middle income countries: there are 12.5 million cases in China, 30M in India and 3.4M in Iran (which is 4% of its population).
These countries have a double problem: on one side they have a huge number of cases. But also, most of their population don’t have easy access to good health care. Because they can’t have their eyes treated early on, the disease progresses and eventually, they need a cornea transplant.
But again, transplantation programs are complex, and they require a lot of infrastructure (tissue banks, hypothermic storage, immunosupression drugs…).
These countries usually don’t have all those means, at least not for the whole population. To give you an idea, there are currently 12.7 million people waiting for a donor cornea, most of them in low-income countries.
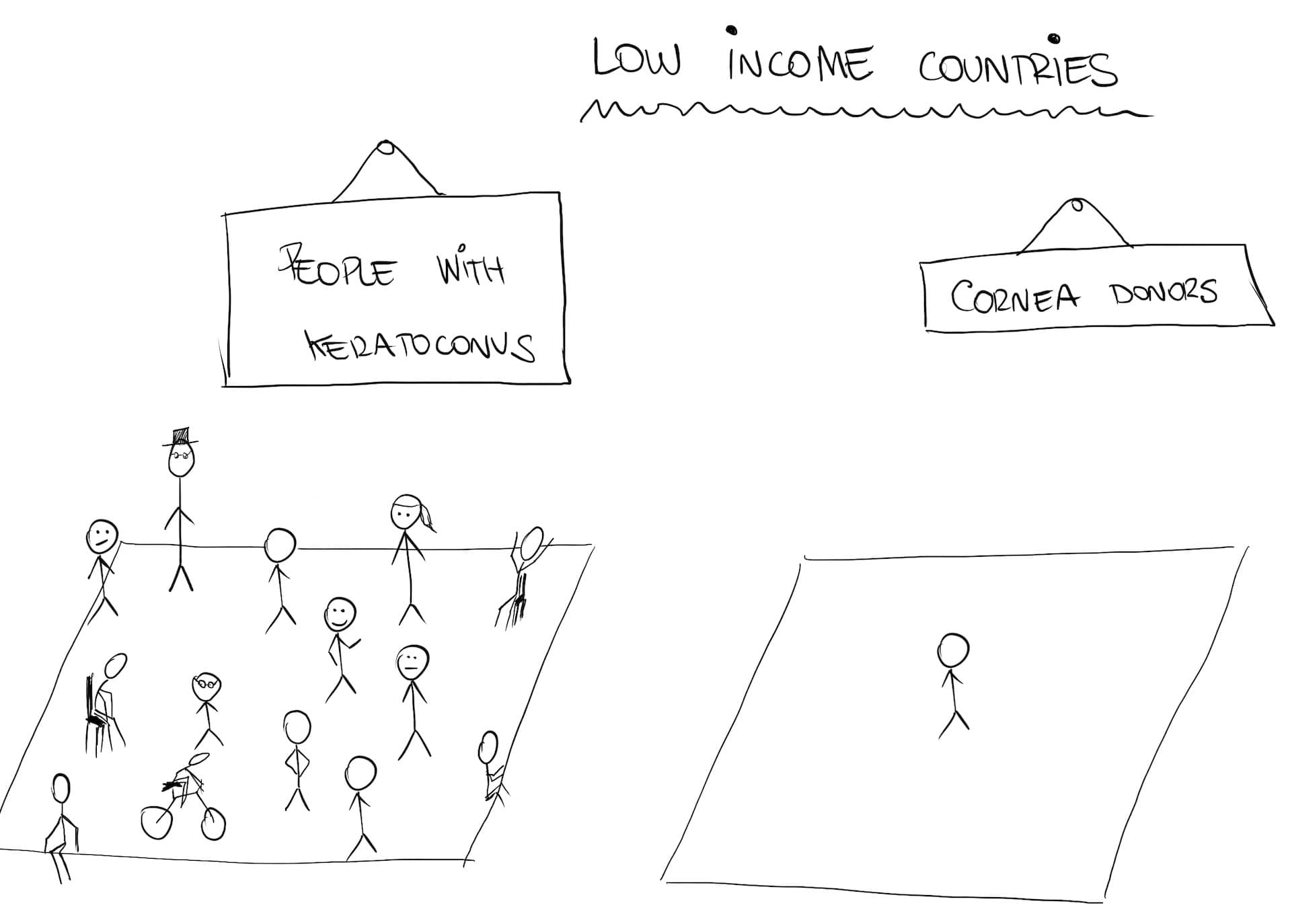
The challenge then, is this: how can we find a cheap and easy way to treat these patients? A way that doesn’t require a cornea transplant?
Fortunately for us, these doctors took up the challenge.
Act #2: the proposal
Basically, what they need to do is come up with some tissue that looks and works like the cornea. It must be transparent and resistant, but still allow for some molding.
“Can we use glass?” you may ask. I don’t think so, because it would break and irritate the tissue around it, causing an inflammatory reaction.
We need some biological tissue, one with no cells inside, otherwise the patient’s body will notice them and reject the tissue.
In 2019, these same scientists already tried something similar. They used single layer collagen sheets. It turned out pretty well, but not great. The problem was that their collagen was too weak, so it required sutures, which in turn caused a strong healing reaction and some of the material kind of melted.
This time, they are going to use a double crosslinked collagen, which is stronger and more resistant. Hopefully, that will solve the problem.
To obtain the collagen sheets, they harvest them from pig skin. Then, they fuse several layers through a dual technique (chemical + photochemical fusion). This generates a thicker, more resistant tissue.
Once they have the collagen disc, it is time to insert it into the cornea and see how it works. Of course, you can’t just test it on humans and see what happens. That’s why there are some previous steps you must follow.
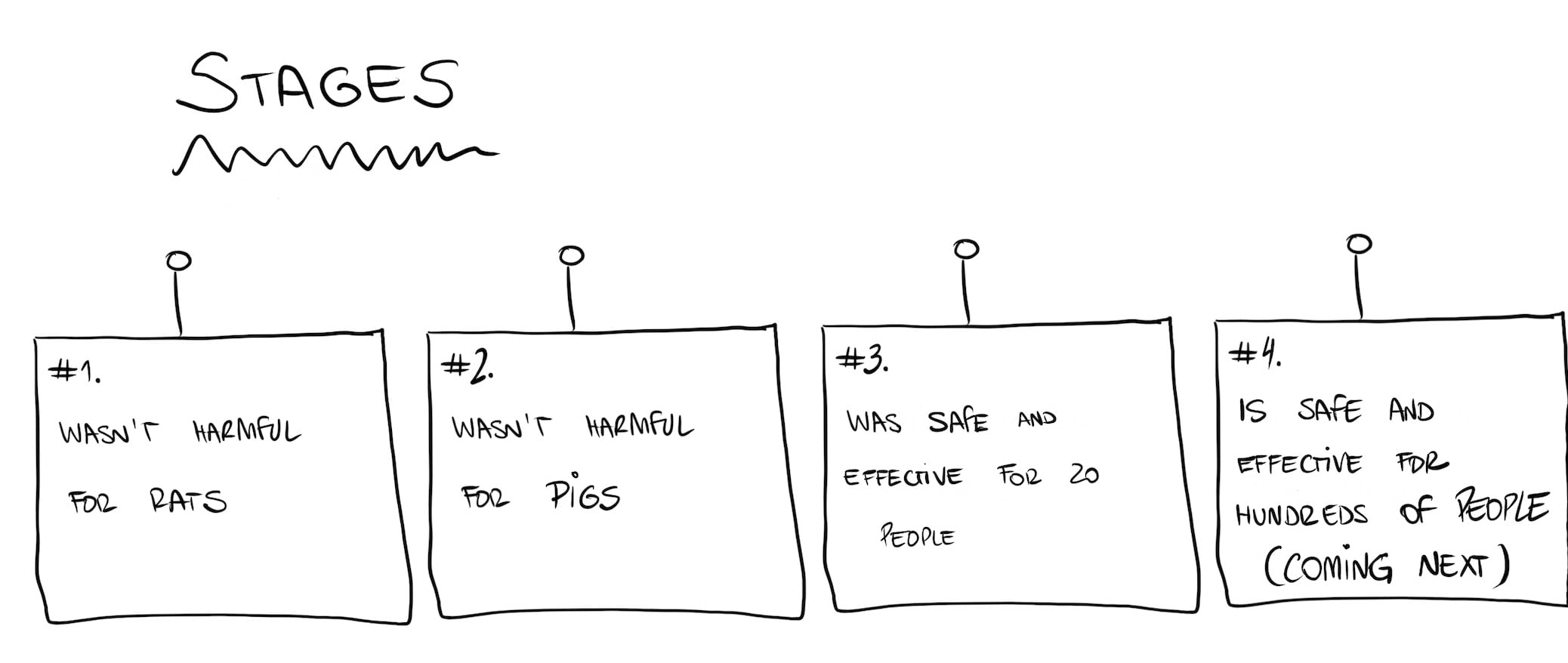
First, you have to make sure the new material is safe. You do this by trying it out with rats. So, they inserted some collagen sheets into the skin of the back of several rats.
Then, they watched them for several days, to see if the wounds were healing or if there were any abnormal reactions. This part was a success: there was no degradation or thinning of the tissue.
Now that we know that the tissue is safe in rats, the second step is check for (further) safety and feasibility. Basically, they need to perform surgery on an animal the same way they intend to do with humans.
They bought 10 minipigs for this, which is a great choice because humans and pigs are surprisingly similar (anatomy-wise). The only problem is they need to find pigs with keratoconus. Because that is an impossible task, they had to choose some healthy pigs and cause them keratoconus.
They did this using a laser, similar to the one they use to treat myopia. Using the laser, they carved the pigs corneas to make them thinner and pointed, obtaining a shape similar to keratoconus. Now they had their “pig-patients” and were ready to go.
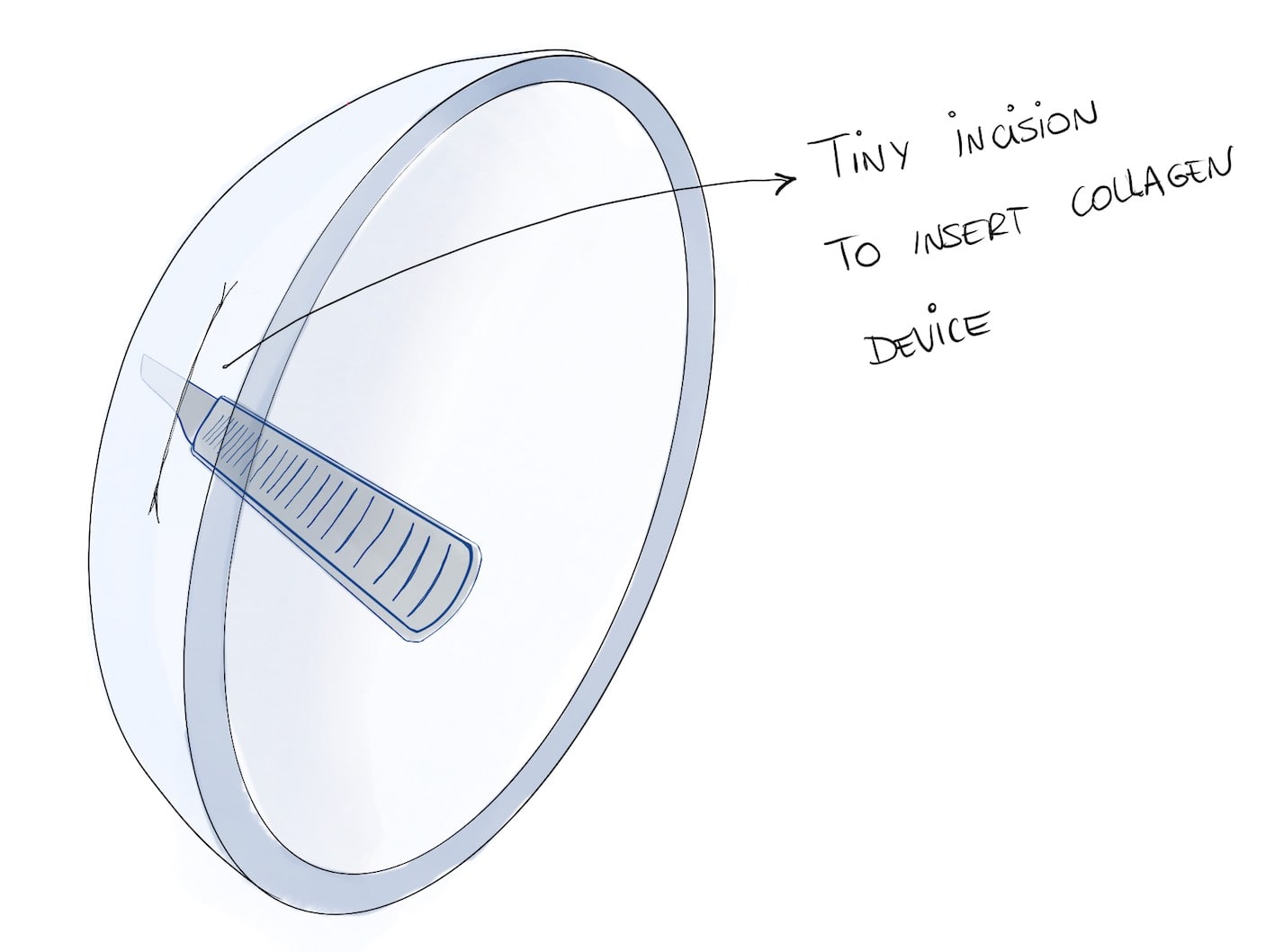
They put the pigs to sleep and started the procedure. They started by making a tiny 7 mm incision on their cornea. Through that slit, they inserted the new collagen sheet, which would do the times of the cornea.
Then they waited for a few days and then checked. Results were good: the material was still transparent and firm. The only problem was with sutures, which again were degrading the tissue. For this reason, they decided to switch to a “no-suture” technique from then on.
Now it was time to move on to the real deal, the first part of human trial. For this stage, they chose a group of 20 patients from Iran and India, due to the lack of other alternatives in these countries.
They carried out the same technique in these patients, only they did not use any sutures this time. Instead, they made a “tiny pocket” inside the thickness of the cornea so that the implant wouldn’t move.
Act #3: the results

2 years have gone by since surgery, and the results are really promising. These are the most impressive results:
-
- 100% of patients improved their sight.
- 4 of them (4/20) now have a perfect vision.
- there were 14 legally-blind people in the study. After the procedure, there were no longer blind patients.
- not a single patient tolerated contact-lenses before the study. All of them (2o) were able to use them afterwards.
- patients had to take immunosuppressant drugs for just 8 weeks. If they had a cornea transplant, they would need them for 2 years.
- there were no significant side effects.
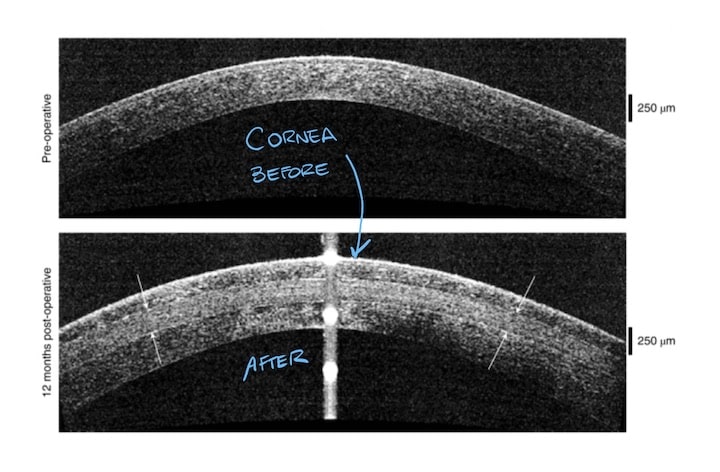
These results are incredible, even more if we consider how this is a first-time procedure. With this discovery, they have opened a new door of opportunities to so many people worldwide. Millions of people may benefit from it, receiving this new therapy in those places where there is no access to a transplant circuit.
However, there are a few steps before we can use this technique worldwide. According to this study the procedure is safe and feasible. We just need some larger clinical trials to make sure it is completely safe and effective.
Leave a Reply